
Chemistry, 25.02.2021 18:40 fluffyanimal456
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation below: Cu + 2AgNO3 →Cu(NO3)2 + 2Ag Calculate the number of moles of copper(II) nitrate produced when 5 moles of copper react. Type your answer as a number with 1 significant figure. Make sure to include the correct units in your answer. Units are a type of measurement i. e. gram (g) or mole (mol). Do not include the chemical formula in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation...
Questions


Biology, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

Health, 04.05.2021 22:00


English, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00


Spanish, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

Mathematics, 04.05.2021 22:00

English, 04.05.2021 22:00

Health, 04.05.2021 22:00

History, 04.05.2021 22:00


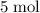 of
of 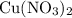 would be produced (assuming that reaction does not run out of
would be produced (assuming that reaction does not run out of 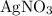 until all the
until all the 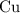 was converted.)
was converted.) 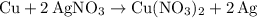 .
. . In other words, the actual equation for this reaction should be:
. In other words, the actual equation for this reaction should be: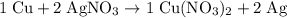 .
.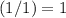 .
.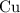 before running out of any other reactant.) This coefficient ratio would be equal to the ratio between:
before running out of any other reactant.) This coefficient ratio would be equal to the ratio between: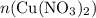 , the number of moles of
, the number of moles of 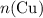 , the number of moles of
, the number of moles of 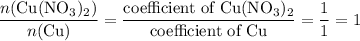 .
.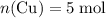 . Assume that
. Assume that 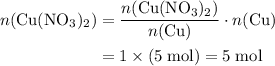 .
.

