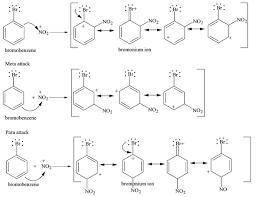
Explain the regiochemical outcome for chlorination of bromobenzene. Ortho attack and para attack are preferred because each of these pathways involves a sigma complex withresonance structures. Attack at the meta position involves formation of a sigma complex with only resonance structures. The reaction will proceed more rapidly via the energy sigma complex, so attack takes place at the ortho and para positions in preference to the meta position.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
Explain the regiochemical outcome for chlorination of bromobenzene.
Ortho attack and para attack ar...
Questions

Biology, 28.03.2021 07:40





History, 28.03.2021 07:40

Engineering, 28.03.2021 07:40

Mathematics, 28.03.2021 07:40


Mathematics, 28.03.2021 07:40


Mathematics, 28.03.2021 07:40

Social Studies, 28.03.2021 07:40

Mathematics, 28.03.2021 07:40

Computers and Technology, 28.03.2021 07:40

Mathematics, 28.03.2021 07:40



Social Studies, 28.03.2021 07:40