Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
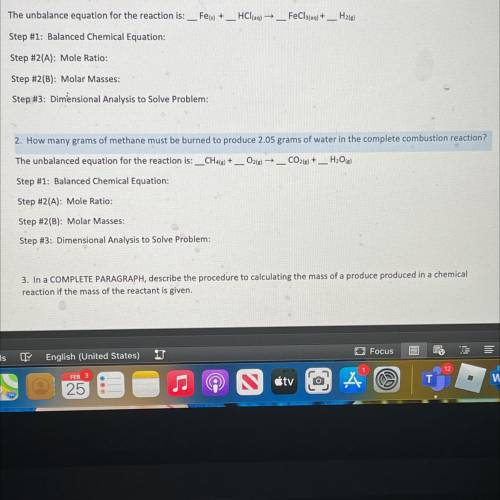
2. How many grams of methane must be burned to produce 2.05 grams of water in the complete combustio...
Questions

History, 03.11.2020 04:50

Mathematics, 03.11.2020 04:50

Mathematics, 03.11.2020 04:50

English, 03.11.2020 04:50

Mathematics, 03.11.2020 04:50


Social Studies, 03.11.2020 04:50


Computers and Technology, 03.11.2020 04:50

Biology, 03.11.2020 04:50

Mathematics, 03.11.2020 04:50

English, 03.11.2020 04:50



Mathematics, 03.11.2020 04:50