
Chemistry, 25.02.2021 14:00 Dreambig85
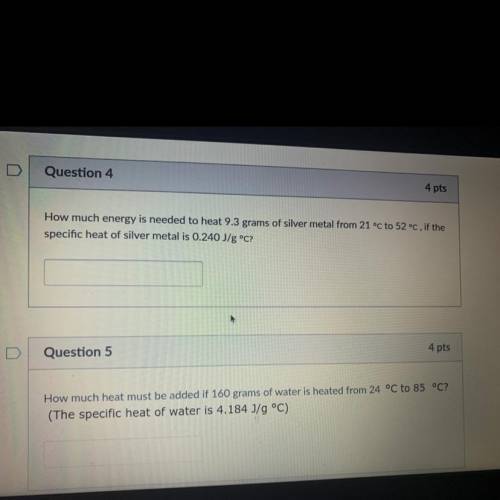
How much energy is needed to heat 9.3 grams of silver metal from 21 °C to 52 °C, if the
specific heat of silver metal is 0.240 J/g °C?
Question 5
4 pts
How much heat must be added if 160 grams of water is heated from 24 °C to 85 °C?
(The specific heat of water is 4.184 J/g °C)
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

You know the right answer?
How much energy is needed to heat 9.3 grams of silver metal from 21 °C to 52 °C, if the
specific he...
Questions


Social Studies, 12.02.2021 02:30

Social Studies, 12.02.2021 02:30




Mathematics, 12.02.2021 02:30





Mathematics, 12.02.2021 02:30



Geography, 12.02.2021 02:30


Mathematics, 12.02.2021 02:30


Mathematics, 12.02.2021 02:30



