
Chemistry, 25.02.2021 06:30 diamondk2019
HELP
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and helium. The partial pressures are
Pnitrogen - 53.0 kPa and Phelium = 25.5 kPa.
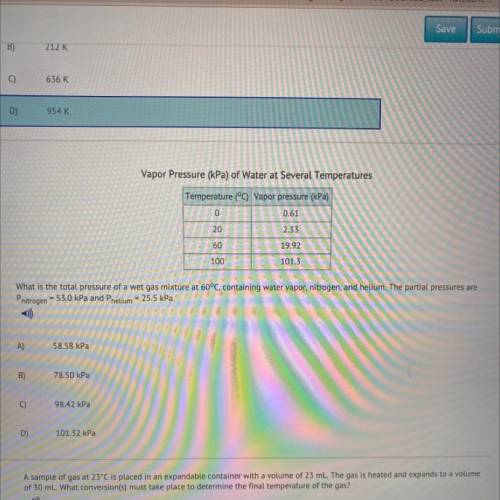

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
HELP
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and...
Questions

Mathematics, 13.02.2020 09:25


History, 13.02.2020 09:26




Mathematics, 13.02.2020 09:27

Mathematics, 13.02.2020 09:27



English, 13.02.2020 09:28

Engineering, 13.02.2020 09:28


Spanish, 13.02.2020 09:29




Health, 13.02.2020 09:29





