
Chemistry, 24.02.2021 21:30 CameronVand21
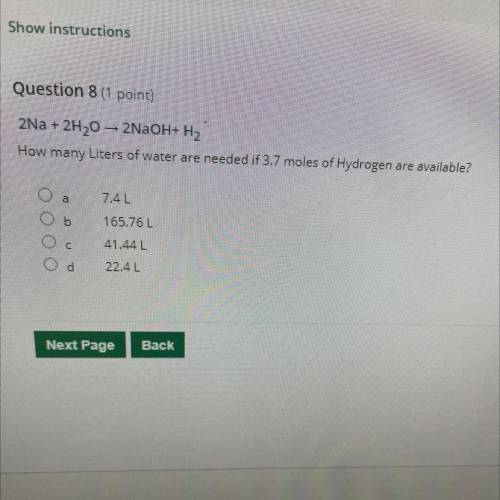
2Na + 2H20 – 2NaOH+H2 How many Liters of water are needed if 3.7 moles of Hydrogen are available?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
2Na + 2H20 – 2NaOH+H2
How many Liters of water are needed if 3.7 moles of Hydrogen are available?
<...
Questions


Chemistry, 11.03.2021 06:20

Mathematics, 11.03.2021 06:20


English, 11.03.2021 06:20

English, 11.03.2021 06:20

Mathematics, 11.03.2021 06:20

Arts, 11.03.2021 06:20

Mathematics, 11.03.2021 06:20


Computers and Technology, 11.03.2021 06:20



Mathematics, 11.03.2021 06:20





French, 11.03.2021 06:20

Physics, 11.03.2021 06:20



