Please help asap
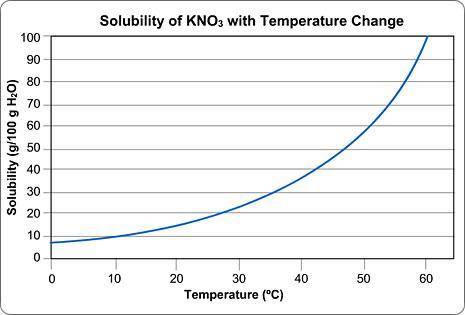
Above is a solubility curve for KNO3.
Solubility has nothing to do wit...

Chemistry, 24.02.2021 04:00 williamslyric
Please help asap
Above is a solubility curve for KNO3.
Solubility has nothing to do with the speed of dissolving; it's a measure of how much salt will dissolve at a given temperature.
The y-axis of the graph shows you how much KNO3 will dissolve in 100 g of water. In other words, it tells you the maximum amount of solute that will dissolve at different temperatures.
The x-axis tells you the minimum temperature needed to dissolve different amounts of KNO3 in 100 g of water.
Approximately how many grams of KNO3 will dissolve in 100 g water at 0 degrees Celsius?
Type in the number only; no units. Round your answer to the nearest whole number.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
You know the right answer?
Questions

Computers and Technology, 12.02.2022 08:20




Social Studies, 12.02.2022 08:20


Mathematics, 12.02.2022 08:20



Social Studies, 12.02.2022 08:20

Mathematics, 12.02.2022 08:20


Mathematics, 12.02.2022 08:20

Biology, 12.02.2022 08:20

Business, 12.02.2022 08:20


Biology, 12.02.2022 08:20

Chemistry, 12.02.2022 08:20

Biology, 12.02.2022 08:20

English, 12.02.2022 08:20



