
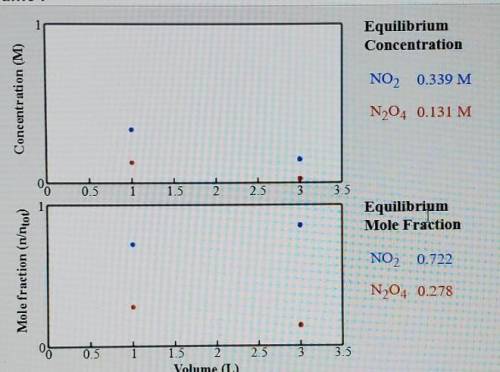
Compare the system (still at 50 C) with a volume of 1.0 L. What is the total amount of gas(mol) present in the container of each of these volumes.
K=0.880 if this is even needed.
I would appreciate just answering the 1.0L with steps so I can work out the second one. Thank you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Compare the system (still at 50 C) with a volume of 1.0 L. What is the total amount of gas(mol) pres...
Questions

Advanced Placement (AP), 12.10.2019 05:10



Biology, 12.10.2019 05:10

Biology, 12.10.2019 05:10


Computers and Technology, 12.10.2019 05:10




Computers and Technology, 12.10.2019 05:10




Computers and Technology, 12.10.2019 05:10




Biology, 12.10.2019 05:10




