
Chemistry, 24.02.2021 02:00 StupidFatChipmunk
For each of the following acid/base reactions, use curved-arrow notation to show the movement of electrons and to predict the products. Label acids and bases as either Bronsted-Lowery or Lewis. I don't understand how to do either someone please explain.
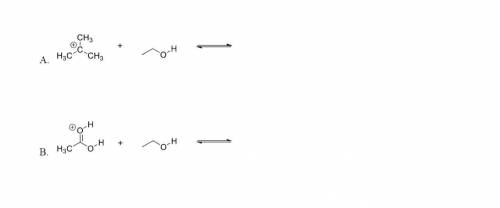

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
For each of the following acid/base reactions, use curved-arrow notation to show the movement of ele...
Questions

Mathematics, 23.12.2020 17:40

Mathematics, 23.12.2020 17:40




Mathematics, 23.12.2020 17:50

Computers and Technology, 23.12.2020 17:50


Mathematics, 23.12.2020 17:50

Mathematics, 23.12.2020 17:50



Mathematics, 23.12.2020 17:50

Mathematics, 23.12.2020 17:50



Mathematics, 23.12.2020 17:50





