
Chemistry, 23.11.2019 06:31 Jennifer16253
To completely neutralize a 0.325 g sample of pure aspirin, 15.50 ml of a sodium hydroxide solution is added. if 16.25 ml of the same sodium hydroxide solution must be added to an aspirin tablet sample during a titration to reach the endpoint, calculate the mass of aspirin in the table
a. 0.310 g
b. 0.288 g
c. 0.392 g
d. 0.450 g
e. 0.341 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
To completely neutralize a 0.325 g sample of pure aspirin, 15.50 ml of a sodium hydroxide solution i...
Questions

Biology, 17.09.2019 17:30


Mathematics, 17.09.2019 17:30


Social Studies, 17.09.2019 17:30


Advanced Placement (AP), 17.09.2019 17:30

Mathematics, 17.09.2019 17:30


Chemistry, 17.09.2019 17:30


Mathematics, 17.09.2019 17:30




English, 17.09.2019 17:30




Physics, 17.09.2019 17:30

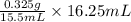 = 0.341 g
= 0.341 g

