
Chemistry, 23.02.2021 21:10 deanlmartin
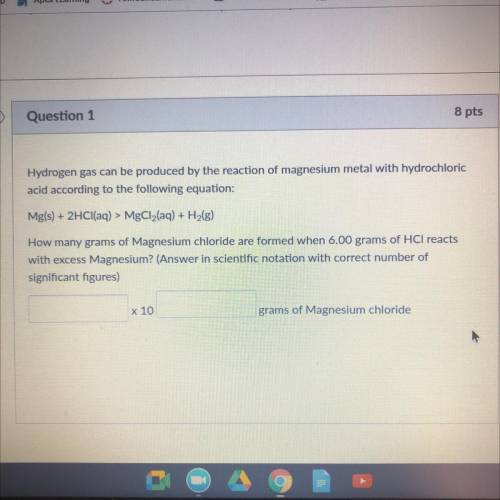
Hydrogen gas can be produced by the reaction of magnesium metal WICH
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are Irmed when 6.00 grams of HCl reacts
with excess Magnesium? (Answer in scientific notation with correct number of
significant figures)
x 10
grams of Magnesium chloride

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2
You know the right answer?
Hydrogen gas can be produced by the reaction of magnesium metal WICH
acid according to the followin...
Questions


Mathematics, 30.06.2021 18:20

History, 30.06.2021 18:20



Mathematics, 30.06.2021 18:20


History, 30.06.2021 18:20

English, 30.06.2021 18:20


Mathematics, 30.06.2021 18:20

History, 30.06.2021 18:20

Social Studies, 30.06.2021 18:20

Mathematics, 30.06.2021 18:20

Computers and Technology, 30.06.2021 18:20


English, 30.06.2021 18:20

English, 30.06.2021 18:20

History, 30.06.2021 18:20



