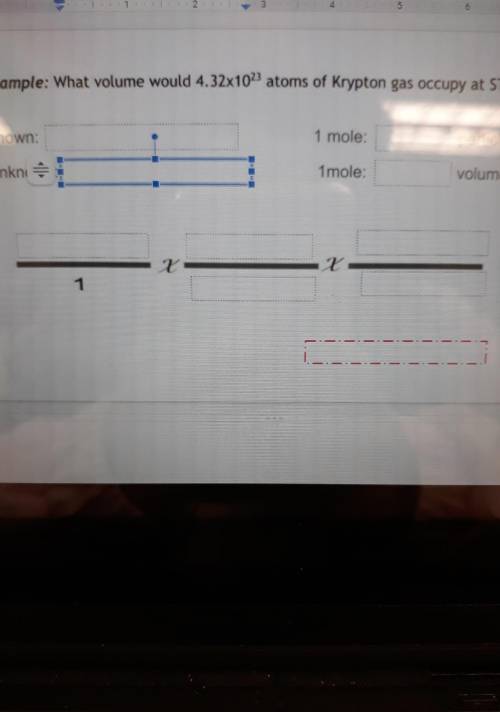
What volume would 4.32x1023 atoms of Krypton gas occupy at STP?
...

Chemistry, 23.02.2021 19:40 Randomtrashygirl
What volume would 4.32x1023 atoms of Krypton gas occupy at STP?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1


Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Questions


Mathematics, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00


History, 20.05.2021 01:00

English, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00

Arts, 20.05.2021 01:00

Mathematics, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00







