
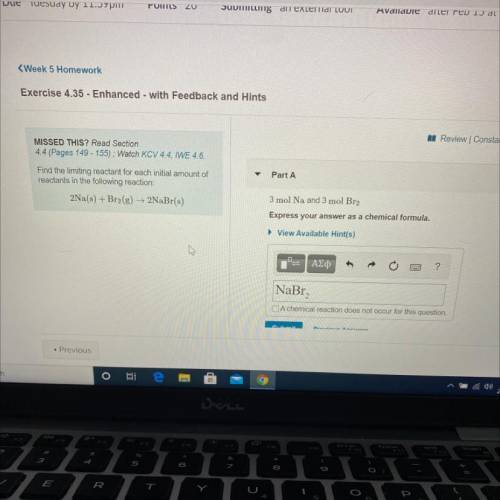
Find the limiting reactant for each initial amount of
reactants in the following reaction: 2Na(s) + Br2(g) + 2NaBr(s)
Part A: 3 mol Na and 3 mol Br2
Part B: 1.6 mol Na and 1.2 mol Br2
Part C: 2.5 mol Na and 1 mol Br2
Part D: 12.9 mol Na and 6.9 mol Br2
Express your answers as a chemical formula.
Please help! will give brainliest

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Find the limiting reactant for each initial amount of
reactants in the following reaction: 2Na(s) +...
Questions

Mathematics, 27.05.2020 05:03



Physics, 27.05.2020 05:03



History, 27.05.2020 05:03

English, 27.05.2020 05:03




English, 27.05.2020 05:03





Mathematics, 27.05.2020 05:03

Mathematics, 27.05.2020 05:03

Mathematics, 27.05.2020 05:03

Mathematics, 27.05.2020 05:03



