
Chemistry, 23.02.2021 04:10 mimiloveyuhh
2C3H6O + 8O2 --> 6CO2 + 6H2O
The combustion reaction for propanol is given above. How many moles of water are produced if 0.15 moles of propanol (C3H6O) reacts with an excess amount oxygen gas?
Please try to show work if possible!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
2C3H6O + 8O2 --> 6CO2 + 6H2O
The combustion reaction for propanol is given above. How many moles...
Questions



Mathematics, 07.11.2020 14:00



History, 07.11.2020 14:00

Mathematics, 07.11.2020 14:00



Social Studies, 07.11.2020 14:00





Mathematics, 07.11.2020 14:00

Computers and Technology, 07.11.2020 14:00



Mathematics, 07.11.2020 14:00

Mathematics, 07.11.2020 14:00

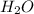 will be produced from 0.15 moles of propanol.
will be produced from 0.15 moles of propanol.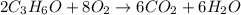
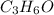 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and 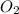 is the excess reagent.
is the excess reagent.
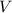 will produce=
will produce=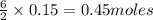 of
of 

