How much energy would be absorbed or released by the h2o in the process 30 grams h2o(
g. at 10...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Questions

Mathematics, 04.06.2020 08:57


Mathematics, 04.06.2020 08:57


Mathematics, 04.06.2020 08:57

English, 04.06.2020 08:57

Mathematics, 04.06.2020 08:57


Health, 04.06.2020 08:57


History, 04.06.2020 08:57




Mathematics, 04.06.2020 08:57

Mathematics, 04.06.2020 08:57

Mathematics, 04.06.2020 08:57

Mathematics, 04.06.2020 08:57

English, 04.06.2020 08:57

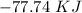
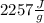 ), so:
), so: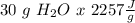
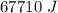
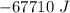
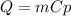 ΔT
ΔT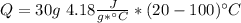
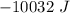
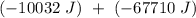
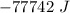 or
or 

