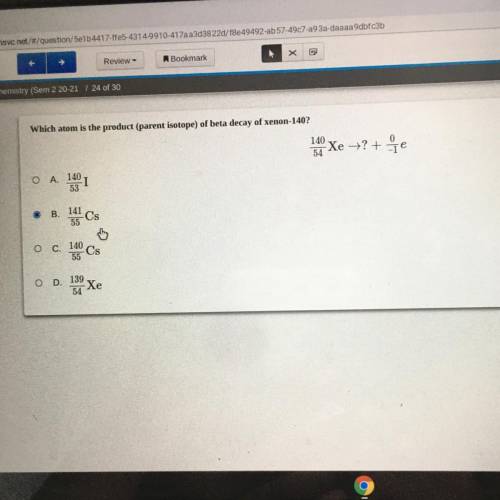
Which atom is the product (parent isotope) of beta decay of xenon-140?
140
Xe = ?+7e
54...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Questions

World Languages, 29.04.2021 23:40



Mathematics, 29.04.2021 23:40

Mathematics, 29.04.2021 23:40

Mathematics, 29.04.2021 23:50

Computers and Technology, 29.04.2021 23:50

Physics, 29.04.2021 23:50


Physics, 29.04.2021 23:50

Mathematics, 29.04.2021 23:50

Mathematics, 29.04.2021 23:50


Mathematics, 29.04.2021 23:50



Physics, 29.04.2021 23:50