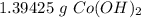Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2Na...

Chemistry, 22.02.2021 23:30 Uhmjujiooo4220
Stoichiometry:
You conduct the following precipitation reaction in a lab:
CoCl₂ + 2NaOH → 2NaCl + Co(OH)₂
If you react 10.0 mL of 1.5 M CoCl₂ with plenty of NaOH, how many grams of Co(OH)₂ will precipitate out?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Questions


Mathematics, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00

English, 28.06.2021 14:00

English, 28.06.2021 14:00


Mathematics, 28.06.2021 14:00

English, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00

English, 28.06.2021 14:00


English, 28.06.2021 14:00

Mathematics, 28.06.2021 14:00



Biology, 28.06.2021 14:00


English, 28.06.2021 14:00

Business, 28.06.2021 14:00

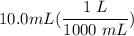 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 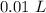 [DA] Find moles of CoCl₂ [Molarity]:
[DA] Find moles of CoCl₂ [Molarity]: 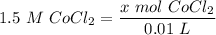 [DA] Solve for x [Multiplication Property of Equality]:
[DA] Solve for x [Multiplication Property of Equality]: 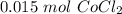 [DA] Set up [Reaction Stoich]:
[DA] Set up [Reaction Stoich]: 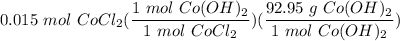 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: