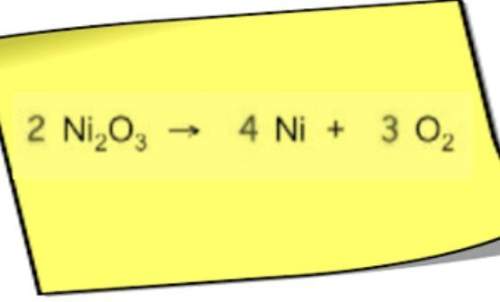
An analytical chemist is titrating 245.6 mL of a 0.6400 M solution of ethylamine (C2H_NH,) with a 0.9000 M solution of HNO3. The pK, of ethylamine is
3.19. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added.
Round your answer to 2 decimal places.
pH ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

You know the right answer?
An analytical chemist is titrating 245.6 mL of a 0.6400 M solution of ethylamine (C2H_NH,) with a 0....
Questions

Mathematics, 22.03.2021 20:00


World Languages, 22.03.2021 20:00



Mathematics, 22.03.2021 20:00

Mathematics, 22.03.2021 20:00

Social Studies, 22.03.2021 20:00

Mathematics, 22.03.2021 20:00



Mathematics, 22.03.2021 20:00


Spanish, 22.03.2021 20:00



Spanish, 22.03.2021 20:00


Mathematics, 22.03.2021 20:00

Mathematics, 22.03.2021 20:00