
Chemistry, 22.02.2021 21:00 KKHeffner02
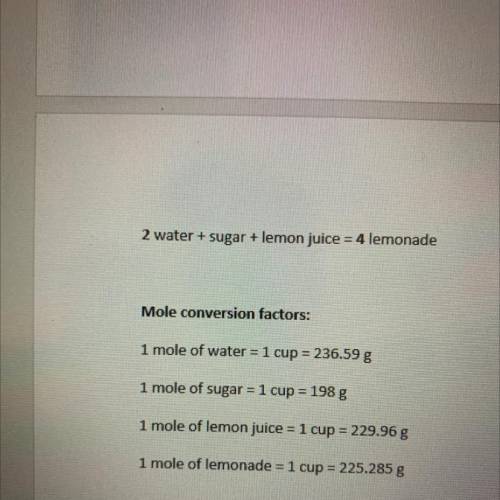
4. Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. Show your stoichiometric calculations below.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
4. Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three...
Questions


Computers and Technology, 31.07.2019 01:00

Mathematics, 31.07.2019 01:00


Biology, 31.07.2019 01:00


Mathematics, 31.07.2019 01:00

Business, 31.07.2019 01:00

Mathematics, 31.07.2019 01:00




Mathematics, 31.07.2019 01:00





Biology, 31.07.2019 01:00

Mathematics, 31.07.2019 01:00

Mathematics, 31.07.2019 01:00



