

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

You know the right answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions

Mathematics, 23.05.2021 20:20


History, 23.05.2021 20:20



Mathematics, 23.05.2021 20:20




Health, 23.05.2021 20:20

Chemistry, 23.05.2021 20:20





Mathematics, 23.05.2021 20:20

English, 23.05.2021 20:20

English, 23.05.2021 20:20

English, 23.05.2021 20:20

Chemistry, 23.05.2021 20:20

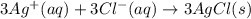
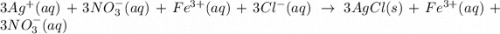
 and
and  are the spectator ions.
are the spectator ions.


