
Chemistry, 22.02.2021 18:50 Svetakotok
A 52.9g sample of brass, which has a specific heat capacity of 0.375·J·g^−1°C^−1, is put into a calorimeter (see sketch at right) that contains 100.0g of water. The temperature of the water starts off at 15.0°C. When the temperature of the water stops changing it's 18.4°C. The pressure remains constant at 1 atm. Calculate the initial temperature of the brass sample.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1
You know the right answer?
A 52.9g sample of brass, which has a specific heat capacity of 0.375·J·g^−1°C^−1, is put into...
Questions

Mathematics, 10.09.2020 17:01

English, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

World Languages, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

Health, 10.09.2020 17:01

Mathematics, 10.09.2020 17:01

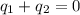
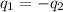
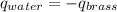
![m_{w}.c_{w}.\Delta T=-[m_{b}.c_{b}.\Delta T]](/tpl/images/1136/4104/e97cd.png)
![100.4.18.(18.4-15)=-[52.9.0.375.(18.4-T)]](/tpl/images/1136/4104/69ce0.png)


