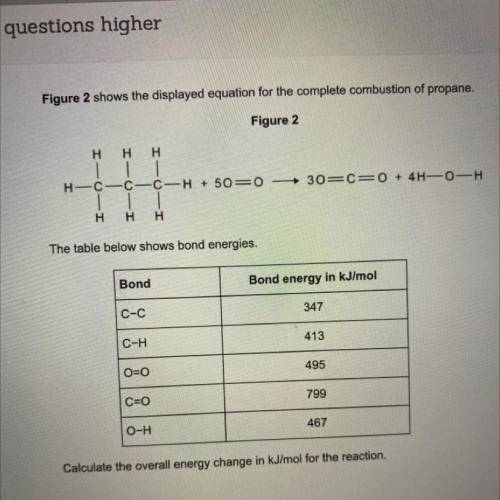
Figure 2 shows the displayed equation for the complete combustion of propane.
Figure 2
Η Η Η<...

Chemistry, 22.02.2021 18:20 ngmasuku3115
Figure 2 shows the displayed equation for the complete combustion of propane.
Figure 2
Η Η Η
30=CEO + 4H-0-H
H-C-C-C-H + 50=0
| |
H Η Η
The table below shows bond energies.
Bond
Bond energy in kJ/mol
C-C
347
C-H
413
O=0
495
C=0
799
O-H
467
Calculate the overall energy change in kJ/mol for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Questions

Mathematics, 13.01.2021 18:10


Mathematics, 13.01.2021 18:20

Mathematics, 13.01.2021 18:20

Mathematics, 13.01.2021 18:20


English, 13.01.2021 18:20

Mathematics, 13.01.2021 18:20






Mathematics, 13.01.2021 18:20

Mathematics, 13.01.2021 18:20







