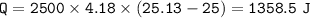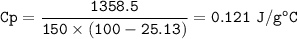Chemistry, 22.02.2021 06:10 lizzyhearts
Use the Gizmo to mix 200 g of granite at 100 °C with 1,000 g of water at 20 °C.
What is the final temperature?
Calculate the temperature change of each substance by subtracting the initial temperature from the final temperature. ∆T water: ∆T granite:
C .How much heat energy (q) did the water gain?
D. Now solve for the specific heat (c) of granite:
E. Repeat steps A through D to find the specific heat (c) of lead:

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1


Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Use the Gizmo to mix 200 g of granite at 100 °C with 1,000 g of water at 20 °C.
What is the final t...
Questions






Mathematics, 27.05.2021 01:00

Biology, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

English, 27.05.2021 01:00





Mathematics, 27.05.2021 01:00




Physics, 27.05.2021 01:00