
Chemistry, 20.02.2021 23:20 rennytheraccoon
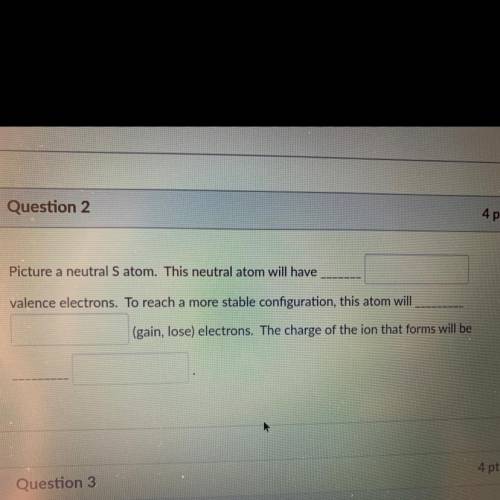
Picture a neutral S atom. This neutral atom will have
valence electrons. To reach a more stable configuration, this atom will
(gain, lose) electrons. The charge of the ion that forms will be
ASAP PLEASE

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Picture a neutral S atom. This neutral atom will have
valence electrons. To reach a more stable con...
Questions

Health, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01


English, 14.10.2020 01:01

English, 14.10.2020 01:01


Mathematics, 14.10.2020 01:01

Social Studies, 14.10.2020 01:01


History, 14.10.2020 01:01

Physics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

English, 14.10.2020 01:01


English, 14.10.2020 01:01


Mathematics, 14.10.2020 01:01




