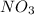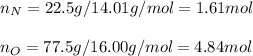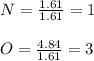A compound is 22.5% nitrogen and 77.5% oxygen, What is the empirical formula of
this compound?...

Chemistry, 20.02.2021 14:10 NeverEndingCycle
A compound is 22.5% nitrogen and 77.5% oxygen, What is the empirical formula of
this compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1


Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Questions

Mathematics, 08.12.2020 02:50

Health, 08.12.2020 02:50

Mathematics, 08.12.2020 02:50

Mathematics, 08.12.2020 02:50


Geography, 08.12.2020 02:50

Mathematics, 08.12.2020 02:50


Business, 08.12.2020 02:50



Mathematics, 08.12.2020 02:50





English, 08.12.2020 02:50


Chemistry, 08.12.2020 02:50

Mathematics, 08.12.2020 02:50