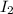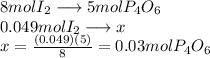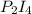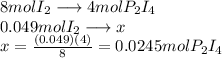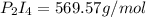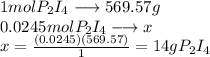Chemistry, 14.10.2019 12:20 kenleighbrooke67
What is the maximum mass of p2i4 that can be prepared from 8.80g of p4o6 and 12.37g of iodine according to the reaction:
5p4o6 +8i2 > 4p2i4 +3p4o10

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
What is the maximum mass of p2i4 that can be prepared from 8.80g of p4o6 and 12.37g of iodine accord...
Questions


Business, 30.06.2021 04:00

Mathematics, 30.06.2021 04:00


History, 30.06.2021 04:00


Mathematics, 30.06.2021 04:00









English, 30.06.2021 04:00

Mathematics, 30.06.2021 04:00

Mathematics, 30.06.2021 04:00

History, 30.06.2021 04:00

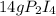
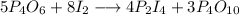
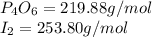
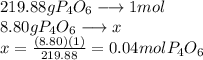

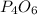 are needed to react 8 moles of
are needed to react 8 moles of