Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
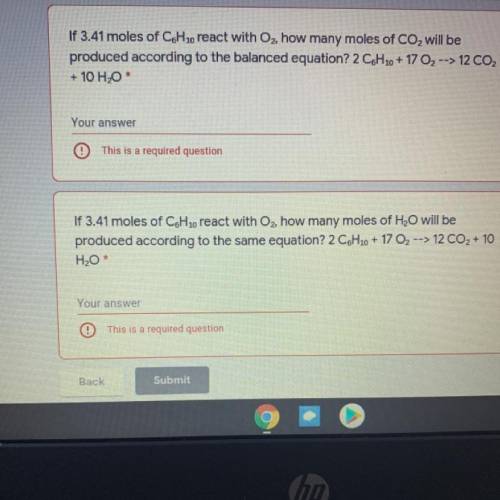
If 3.41 moles of C6H 10 react with Oz, how many moles of CO2 will be
produced according to the bala...
Questions

Mathematics, 23.04.2020 21:21

Chemistry, 23.04.2020 21:21



Mathematics, 23.04.2020 21:21

Biology, 23.04.2020 21:21


Biology, 23.04.2020 21:21

Mathematics, 23.04.2020 21:21

History, 23.04.2020 21:21


Computers and Technology, 23.04.2020 21:21

Mathematics, 23.04.2020 21:21



Biology, 23.04.2020 21:21

Health, 23.04.2020 21:21

Mathematics, 23.04.2020 21:21