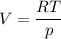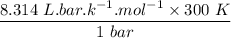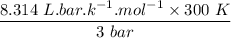Chemistry, 19.02.2021 17:00 bunbun2913
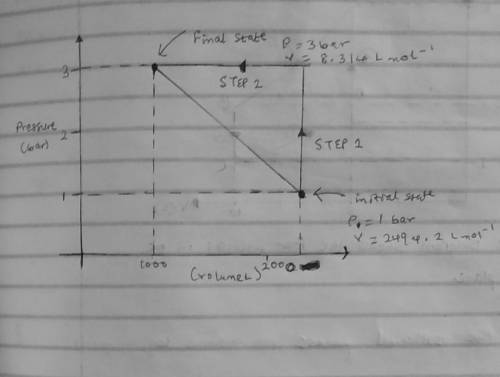
An ideal gas that is confined in piston-cylinder assembly (i. e., closed system) goes from an initial state of 1 bar at 300 K to a final state of 3 bar at 300 K by the following two-step process.
Process Path.
(Step 1) Heating at constant volume, and then
( Step 2) Cooling by holding the pressure constant.
Required:
a. Determine the initial and final molar mass.
b. Illustrate the two paths on a pressure-volume diagram. Clearly label the initial and final states, process steps and the direction of each step in the diagram.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
An ideal gas that is confined in piston-cylinder assembly (i. e., closed system) goes from an initia...
Questions

Mathematics, 20.07.2019 06:50






Mathematics, 20.07.2019 06:50

Geography, 20.07.2019 07:00



Mathematics, 20.07.2019 07:00



Social Studies, 20.07.2019 07:00

Mathematics, 20.07.2019 07:00

Health, 20.07.2019 07:00


Mathematics, 20.07.2019 07:00

History, 20.07.2019 07:00

History, 20.07.2019 07:00