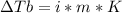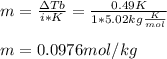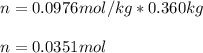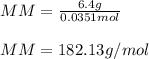Chemistry, 19.02.2021 09:30 marlandwilliams10
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0.49 K higher than the boiling point of pure CC14. Calculate the molar mass of
adrenaline. (K = 5.02 kg K/mol).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

You know the right answer?
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0...
Questions

Mathematics, 22.06.2021 17:40

Mathematics, 22.06.2021 17:40

Mathematics, 22.06.2021 17:40

Mathematics, 22.06.2021 17:40



Mathematics, 22.06.2021 17:40



Mathematics, 22.06.2021 17:40

English, 22.06.2021 17:40



Mathematics, 22.06.2021 17:40

Mathematics, 22.06.2021 17:40

History, 22.06.2021 17:40




Mathematics, 22.06.2021 17:40