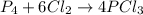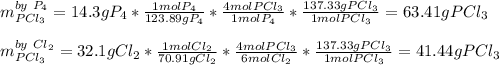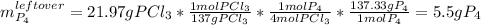For the following reaction, 14.3 grams of phosphorus (P4) are allowed to react with 32.1 grams of chlorine gas. phosphorus (P4) (s) + chlorine (g) phosphorus trichloride (l) What is the maximum amount of phosphorus trichloride that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

You know the right answer?
For the following reaction, 14.3 grams of phosphorus (P4) are allowed to react with 32.1 grams of ch...
Questions


History, 27.01.2021 20:50

Mathematics, 27.01.2021 20:50


Mathematics, 27.01.2021 20:50

Mathematics, 27.01.2021 20:50

History, 27.01.2021 20:50


Mathematics, 27.01.2021 20:50



Mathematics, 27.01.2021 20:50


Geography, 27.01.2021 20:50


Mathematics, 27.01.2021 20:50

World Languages, 27.01.2021 20:50

Mathematics, 27.01.2021 20:50


Mathematics, 27.01.2021 20:50