Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

You know the right answer?
4 OT 5
A 35g chunk of metal at 130C was dropped in a bucket containing 220g of water at 25C. The fi...
Questions

Spanish, 19.05.2020 15:24


History, 19.05.2020 15:25


Biology, 19.05.2020 15:25

Mathematics, 19.05.2020 15:25

Mathematics, 19.05.2020 15:25


English, 19.05.2020 15:25



Advanced Placement (AP), 19.05.2020 15:25


History, 19.05.2020 15:25

Mathematics, 19.05.2020 15:25

Mathematics, 19.05.2020 15:25


Mathematics, 19.05.2020 15:25

History, 19.05.2020 15:25

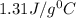
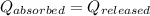
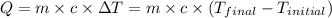
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/1127/3997/b0e58.png)
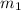 = mass of metal = 35 g
= mass of metal = 35 g
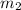 = mass of water = 220 g
= mass of water = 220 g
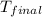 = final temperature =
= final temperature = 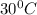
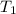 = temperature of metal =
= temperature of metal = 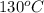
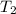 = temperature of water =
= temperature of water = 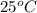
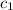 = specific heat of metal = ?
= specific heat of metal = ?
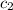 = specific heat of water =
= specific heat of water = 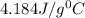
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1127/3997/09236.png)
![35\times c_1\times (30-130)^0C=-[220g\times 4.184\times (30-25)]](/tpl/images/1127/3997/3cabe.png)