
1.61 x 104 molecules
Od
2.26 x 1029 molecules
Oe
1.77 x 1029 molecules
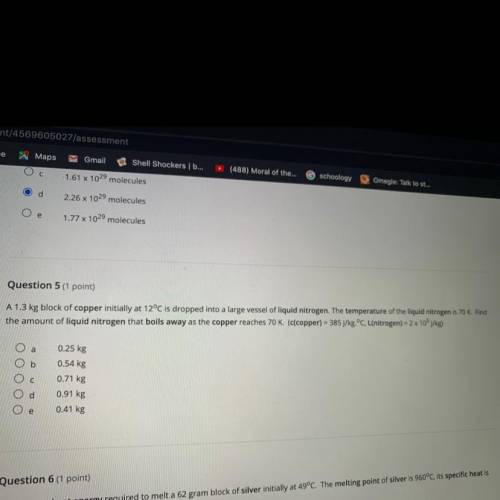
Question 5 (1 point)
A 1.3 kg block of copper initially at 12°C is dropped into a large vessel of liquid nitrogen. The temperature of the liquid nitrogen is 70 K. Find
the amount of liquid nitrogen that boils away as the copper reaches 70 K. (c(copper) = 385 J/kg.°C, L(nitrogen) = 2 x 105 j/kg

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

You know the right answer?
1.61 x 104 molecules
Od
2.26 x 1029 molecules
Oe
1.77 x 1029 molecules
Ques...
2.26 x 1029 molecules
Oe
1.77 x 1029 molecules
Ques...
Questions

Business, 09.03.2021 02:10



Mathematics, 09.03.2021 02:10

Mathematics, 09.03.2021 02:10






Mathematics, 09.03.2021 02:10


Geography, 09.03.2021 02:10

Mathematics, 09.03.2021 02:10

Mathematics, 09.03.2021 02:10


Mathematics, 09.03.2021 02:10



Advanced Placement (AP), 09.03.2021 02:10



