
Chemistry, 17.02.2021 21:40 emblemhacks
A. Calculate the percentage yield for SiO2 for trial 1. Also, determine the leftover reactant for the trial. Show your Work. b. Based on the percentage yield in trial 2, explain what ratio of reactants is more efficient for the given reaction.
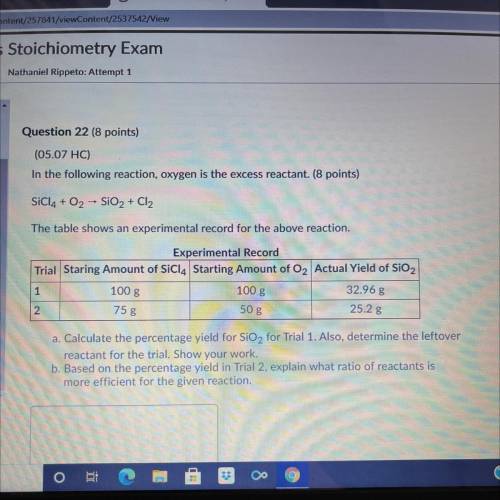

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
A. Calculate the percentage yield for SiO2 for trial 1. Also, determine the leftover reactant for th...
Questions

Social Studies, 03.12.2019 01:31


Mathematics, 03.12.2019 01:31


Mathematics, 03.12.2019 01:31

History, 03.12.2019 01:31


English, 03.12.2019 01:31

Mathematics, 03.12.2019 01:31

Mathematics, 03.12.2019 01:31


English, 03.12.2019 01:31




History, 03.12.2019 01:31






