Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
You know the right answer?
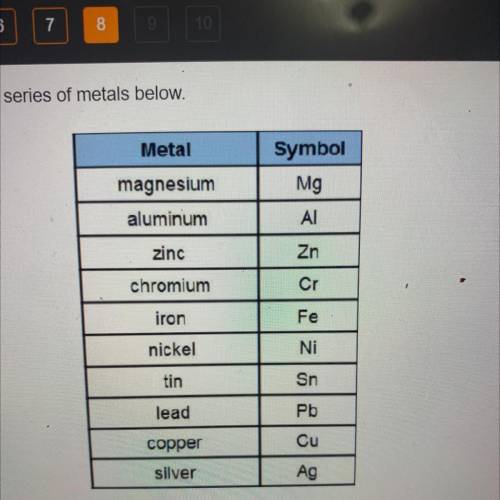
The following reaction occurs in an electrochemical cell.
Sn2++ Pb → Pb2++ Sn
What type of el...
What type of el...
Questions


Social Studies, 09.04.2021 20:10

Mathematics, 09.04.2021 20:10


Mathematics, 09.04.2021 20:10

Mathematics, 09.04.2021 20:10




Mathematics, 09.04.2021 20:10



Social Studies, 09.04.2021 20:10


Mathematics, 09.04.2021 20:10


English, 09.04.2021 20:10



Chemistry, 09.04.2021 20:10