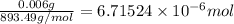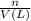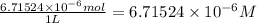Chemistry, 14.10.2019 05:30 skylerdemi1
So my chem experiment asks us: what is the molarity of a 6.0 ppm solution (it's a 6.0 ppm chlorophyll solution, hexane is the solvent)
now, i've found a few ways to solve for it but they both give me different values. if it's possible, could you just tell me which is right.
#1 way: 6parts/1million --> 0.006g/l --> take 0.006g convert w/ molar mass chlorophyll (1 mole/ 893.49 g) --> 6.71524 *10^(-6) moles
put 6.71524 *10^(-6) over 1l --> 6.71524 *10^(-6) m
0r 6parts/1million --> 6g/1000,000g --> take 6g convert w/ molar mass chlorophyll (1 mole/ 893.49 g) --> 6.71524 *10^(-3) moles
use hexane's density to convert 1000,000g to (ml first then) l (density: 1ml/0.6548g) --> 1527.18388 l
put 6.71524 *10^(-3) moles over 1527.18388 l --> 4.397*10^(-6) m
or 6parts/1million --> 6g/1000,000g take 6g convert w/ molar mass chlorophyll (1 mole/ 893.49 g) --> 6.71524 *10^(-3) moles
put 6.71524 *10^(-3) moles over 1000,000 --> 6.71524 *10^(-9) m
i think the second one is the right one but i'm not sure. could you me? !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
So my chem experiment asks us: what is the molarity of a 6.0 ppm solution (it's a 6.0 ppm chlorophy...
Questions

Computers and Technology, 09.10.2019 17:30

Physics, 09.10.2019 17:30

Geography, 09.10.2019 17:30


History, 09.10.2019 17:30






English, 09.10.2019 17:30



Health, 09.10.2019 17:30

Physics, 09.10.2019 17:30



English, 09.10.2019 17:30