
Chemistry, 17.02.2021 01:10 gui00g7888888888888
For the reaction below, the order of reaction for NH4+ is 1, and the order of reaction for NO2 is 1.
NH4(aq) + NO3(aq) = N2(g) + 2H2O(1)
What is the rate law for the reaction?
A. R=k[NH+4] [NO-2]
B. R= [NH+4] [NO-2]2
C. R=k[NH]4 [NO]2
D. R=K[N2][H20]
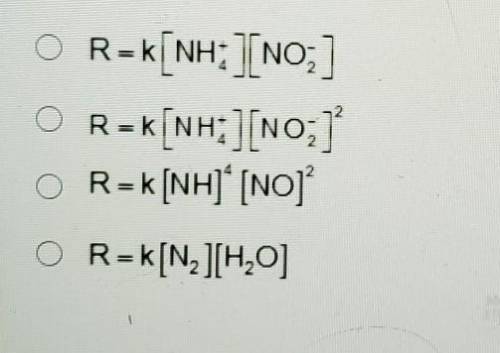

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
For the reaction below, the order of reaction for NH4+ is 1, and the order of reaction for NO2 is 1....
Questions


Mathematics, 05.02.2020 10:51

History, 05.02.2020 10:51

Computers and Technology, 05.02.2020 10:51

History, 05.02.2020 10:51



Biology, 05.02.2020 10:51

Computers and Technology, 05.02.2020 10:51





Mathematics, 05.02.2020 10:51

Mathematics, 05.02.2020 10:51

English, 05.02.2020 10:51


Mathematics, 05.02.2020 10:51

Mathematics, 05.02.2020 10:51




