
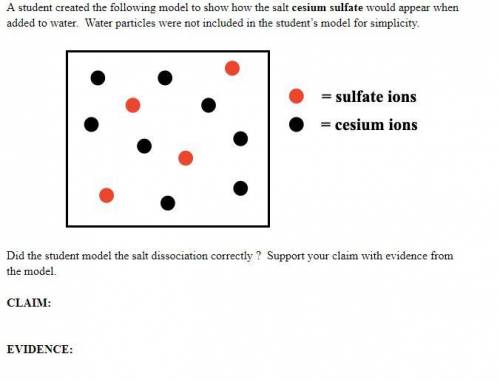
A student created the following model to show how the salt cesium sulfate would appear when added to water. Water particles were not included in the student’s model for simplicity. Did the student model the salt dissociation correctly ? Support your claim with evidence from the model.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
A student created the following model to show how the salt cesium sulfate would appear when added to...
Questions



Mathematics, 30.09.2019 12:10

Biology, 30.09.2019 12:10

Business, 30.09.2019 12:10


Biology, 30.09.2019 12:10


Biology, 30.09.2019 12:10

Mathematics, 30.09.2019 12:10


Mathematics, 30.09.2019 12:10

Arts, 30.09.2019 12:10

History, 30.09.2019 12:10





History, 30.09.2019 12:10



