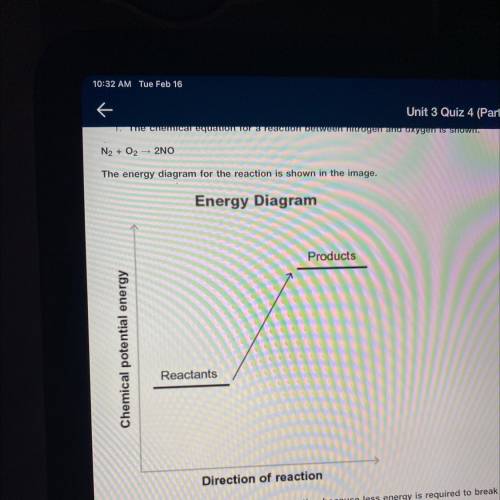
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
T...

Chemistry, 16.02.2021 21:50 brookie125
The chemical equation for a reaction between nitrogen and oxygen is shown.
N2+ O2 —> 2NO
Then energy diagram for the reaction is shown in the image.
A) energy is absorbed, less energy, break bonds, form new bonds.
B) energy is released, more energy, break bonds, form new bonds
C) Energy is absorbed, the bond energy of the reactant is higher than the bond energy of the products
D) Energy is released, the bond energy of the reactant is lower than the bond energy of the products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Questions

English, 03.06.2021 20:10






Physics, 03.06.2021 20:10

Mathematics, 03.06.2021 20:10



Mathematics, 03.06.2021 20:10

Mathematics, 03.06.2021 20:10



Mathematics, 03.06.2021 20:20



Chemistry, 03.06.2021 20:20


Mathematics, 03.06.2021 20:20



