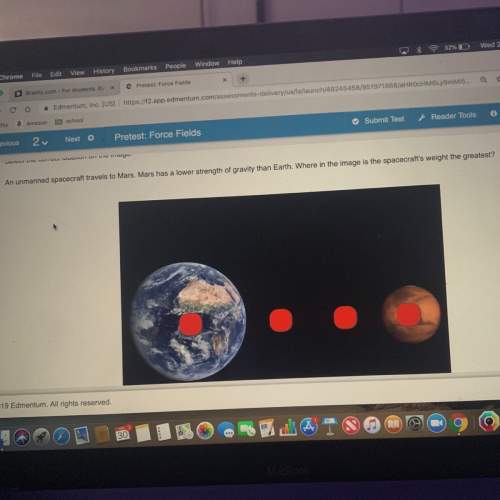
2. A solubilidade do hidróxido de cálcio é de 1,6 g/dm3,
à temperatura de 20 °C.
a. Indica o s...

Chemistry, 16.02.2021 19:20 liaholmes8
2. A solubilidade do hidróxido de cálcio é de 1,6 g/dm3,
à temperatura de 20 °C.
a. Indica o significado da afirmação anterior.
b. Qual é a massa de hidróxido de cálcio que é
possível dissolver em 50 cm de água?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Questions

English, 03.12.2019 15:31


Mathematics, 03.12.2019 15:31

Mathematics, 03.12.2019 15:31

English, 03.12.2019 15:31

Physics, 03.12.2019 15:31



Spanish, 03.12.2019 15:31

Social Studies, 03.12.2019 15:31


Mathematics, 03.12.2019 15:31

History, 03.12.2019 15:31

Mathematics, 03.12.2019 15:31


English, 03.12.2019 15:31


World Languages, 03.12.2019 15:31