
Chemistry, 16.02.2021 18:50 screamqueen
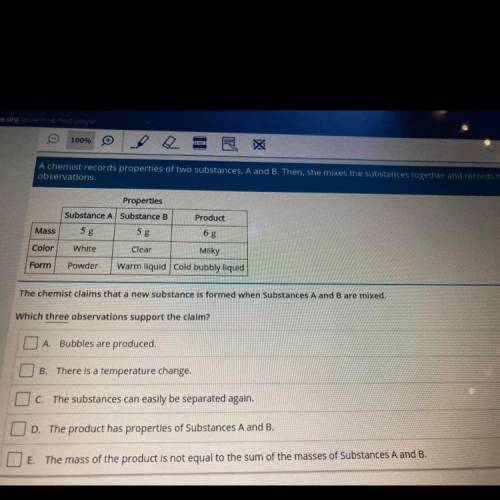
A chemist records properties of two substances, A and B. Then, she mixes the substances together and records her
observations.
Product
Properties
Substance A Substance B
5 g
5 g
White
Clear
Mass
Color
Milky
Form
Powder
Warm liquid cold bubbly liquid
The chemist claims that a new substance is formed when Substances A and B are mixed.
Which three observations support the claim?
O A Bubbles are produced.
B
There is a temperature change.
O c. The substances can easily be separated again.
OD. The product has properties of Substances A and B.
O E. The mass of the product is not equal to the sum of the masses of Substances A and B.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
A chemist records properties of two substances, A and B. Then, she mixes the substances together and...
Questions

Mathematics, 27.01.2020 05:31

Mathematics, 27.01.2020 05:31


Mathematics, 27.01.2020 05:31


History, 27.01.2020 05:31


Biology, 27.01.2020 05:31


Mathematics, 27.01.2020 05:31

Mathematics, 27.01.2020 05:31

Computers and Technology, 27.01.2020 05:31



Mathematics, 27.01.2020 05:31

Business, 27.01.2020 05:31

Chemistry, 27.01.2020 05:31


Mathematics, 27.01.2020 05:31



