
. Carbamide (CH4N2O), can be synthesized by the reaction of ammonia (NH3) with carbon
dioxide (CO2):
2 NH3 (aq) + CO2 (aq) CH4N2O (aq) + H2O (l)
An industrial synthesis of urea obtains 87.5 kg of urea upon reaction of 68.2 kg of ammonia
with 105 kg of carbon dioxide. Determine the limiting reactant, theoretical yield of urea and percent yeild for the reaction

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
. Carbamide (CH4N2O), can be synthesized by the reaction of ammonia (NH3) with carbon
dioxide (CO2)...
Questions


Mathematics, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00






Mathematics, 05.03.2021 01:00


Physics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00




Mathematics, 05.03.2021 01:00

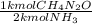 = 2.00 kmol CH₄N₂O
= 2.00 kmol CH₄N₂O

