Chemistry, 16.02.2021 04:20 baileyflemingde
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How many grams of sodium azide are required to produce 24.4 L of nitrogen gas at standard temperature and pressure? 2NaN3 --> 2Na + 3N2
47.2 g of sodium azide
106.2 g of sodium azide
1.63 g of sodium azide
0.726 g of sodium azide

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How...
Questions

Advanced Placement (AP), 16.04.2021 20:00




Biology, 16.04.2021 20:00

Biology, 16.04.2021 20:00

Mathematics, 16.04.2021 20:00

Mathematics, 16.04.2021 20:00

Chemistry, 16.04.2021 20:00

Mathematics, 16.04.2021 20:00


Mathematics, 16.04.2021 20:00

Chemistry, 16.04.2021 20:00

Law, 16.04.2021 20:00


Chemistry, 16.04.2021 20:00

History, 16.04.2021 20:00


Chemistry, 16.04.2021 20:00

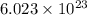 of particles.
of particles.
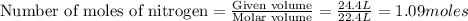
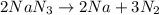
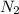 are produced by = 2 moles of
are produced by = 2 moles of 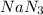
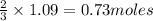 of
of