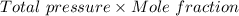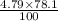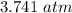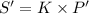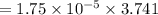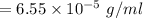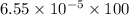Chemistry, 15.02.2021 22:20 giraffesaur44
Nitrogen, , is soluble in blood and can cause intoxication at sufficient concentration. For this reason, the U. S. Navy advises divers using compressed air not to go below 125 feet. The total pressure at this depth is 4.79 atm. If the solubility of nitrogen at 1.00 atm is g/100 mL of water, and the mole percent of nitrogen in air is 78.1, what is the solubility of nitrogen in water from air at 4.79 atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1


Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Nitrogen, , is soluble in blood and can cause intoxication at sufficient concentration. For this rea...
Questions


Biology, 17.12.2020 01:30

Mathematics, 17.12.2020 01:30


Mathematics, 17.12.2020 01:30


Mathematics, 17.12.2020 01:30




Mathematics, 17.12.2020 01:30

English, 17.12.2020 01:30



Mathematics, 17.12.2020 01:30


Biology, 17.12.2020 01:30

Mathematics, 17.12.2020 01:30

Biology, 17.12.2020 01:30

Mathematics, 17.12.2020 01:30

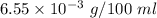 "
"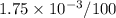
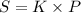
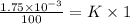
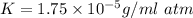 (Henry's constant)
(Henry's constant)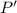 ) will be:
) will be: