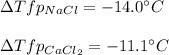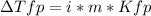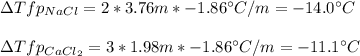Chemistry, 15.02.2021 20:10 battlemarshmell
Sodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride (CaCl2) is sometimes used for this purpose too. Let us compare the effectiveness of equal masses of these two compounds in lowering the freezing point of water by calculating the freezing point depression of solutions containing 220. g of each salt in 1.00 kg of water. (An advantage of is that it acts more quickly because it is hygroscopic, that is, it absorbs moisture from the air to create a solution and begin the process. A disadvantage is that this compound is more costly.) Assume full dissociation of ionic compounds. Kfp(H2O)= -1.86 °C/m.
ΔTfp= °C for NaCl
ΔTfp= °C for CaCl2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
Sodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride (Ca...
Questions

English, 06.10.2021 17:10

History, 06.10.2021 17:10

Chemistry, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10

English, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10



Social Studies, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10

Physics, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10

History, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10


Mathematics, 06.10.2021 17:10

Mathematics, 06.10.2021 17:10


Social Studies, 06.10.2021 17:10