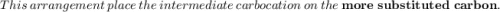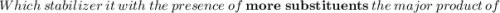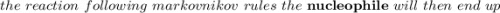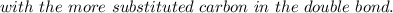Chemistry, 15.02.2021 19:50 elissadiaz15
According to Markovnikov's rule of the electrophilic addition to an alkene, the electrophile, usually a proton, is more likely to add to the in a double bond. This arrangement places the intermediate carbocation on the , which stabilizes it with the presence of . In the major product of a reaction following Markovnikov's rule, the will then end up on the more-substituted carbon in a double

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
According to Markovnikov's rule of the electrophilic addition to an alkene, the electrophile, usuall...
Questions



Mathematics, 05.05.2020 01:22


Mathematics, 05.05.2020 01:22

Biology, 05.05.2020 01:22

Mathematics, 05.05.2020 01:22


Mathematics, 05.05.2020 01:22






Mathematics, 05.05.2020 01:22

Biology, 05.05.2020 01:22



Mathematics, 05.05.2020 01:23

French, 05.05.2020 01:23