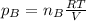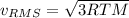Chemistry, 15.02.2021 19:40 mikehager4321
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Which of the two gases exerts the greater partial pressure?
b. The molecules or atoms of which gas have the greater average velocity?
c. The molecules of which gas have the greater average kinetic energy?
d . If a small hole were opened in the flask, which gas effuses more quickly?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2

Chemistry, 23.06.2019 19:30
There was a water with a boat and a rock in a boat. will the level of the water stay the same or higher or lower if u remove the rock from the boat and put it under water? ?
Answers: 3

Chemistry, 23.06.2019 19:40
The reaction: (ch3)3cbr + oh− → (ch3)3coh + br− in a certain solvent is first order with respect to oh−. in several experiments, the rate constant k was determined at different temperatures. a plot of ln(k) vs 1/t was constructed and the slope of the line was -1.10 x 104 k with a y-intercept of 33.5. what is the value for k at a temperature of 25°c?
Answers: 1
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Whi...
Questions

Physics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50


Mathematics, 12.10.2019 14:50




Business, 12.10.2019 14:50

Biology, 12.10.2019 14:50


Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50



World Languages, 12.10.2019 14:50