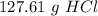Chemistry, 15.02.2021 04:10 gulleraliyeva1999
one reaction that produces hydrogen gas can be represented by the unbalanced chemical equation Mg(s)+HCI(aq) -> MgCI(aq)+H2(g). What is the mass of HCI is consumed by the reaction of 3.25 mol of magnesium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2


Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1
You know the right answer?
one reaction that produces hydrogen gas can be represented by the unbalanced chemical equation Mg(s)...
Questions



Mathematics, 27.10.2021 09:30

English, 27.10.2021 09:30


Mathematics, 27.10.2021 09:30

Mathematics, 27.10.2021 09:30



Mathematics, 27.10.2021 09:30

Health, 27.10.2021 09:30

History, 27.10.2021 09:30

Mathematics, 27.10.2021 09:30


English, 27.10.2021 09:30


World Languages, 27.10.2021 09:30


Social Studies, 27.10.2021 09:30

Biology, 27.10.2021 09:30

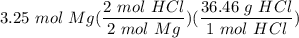 [S - DA] Multiply/Divide [Cancel out units]:
[S - DA] Multiply/Divide [Cancel out units]: