
Chemistry, 13.02.2021 19:20 krobinson27
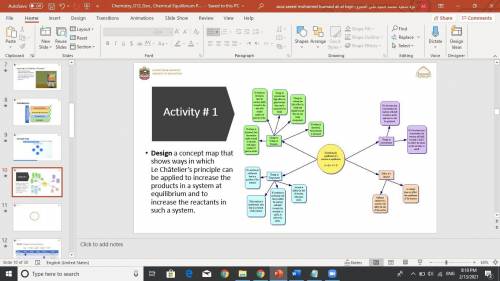
Design a concept map that shows ways in which Le Châtelier’s principle can be applied to increase the products in a system at equilibrium and to increase the reactants in such a system. can i please have it now?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

You know the right answer?
Design a concept map that shows ways in which Le Châtelier’s principle can be applied to increase th...
Questions

Mathematics, 11.04.2021 20:50


Social Studies, 11.04.2021 20:50

Social Studies, 11.04.2021 20:50


Mathematics, 11.04.2021 20:50



Chemistry, 11.04.2021 21:00

Mathematics, 11.04.2021 21:00


Biology, 11.04.2021 21:00

Chemistry, 11.04.2021 21:00



Mathematics, 11.04.2021 21:00

English, 11.04.2021 21:00



Chemistry, 11.04.2021 21:00



