
Chemistry, 13.02.2021 09:40 cjasmine626
If 175 g of phosphoric acid reacts with 150.0 g of sodium hydroxide, what is the limiting reactant? How many grams of sodium phosphate will produced? How many grams of excess reactant will be left over?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
If 175 g of phosphoric acid reacts with 150.0 g of sodium hydroxide, what is the limiting reactant?...
Questions



History, 28.06.2019 09:10

Mathematics, 28.06.2019 09:10

Mathematics, 28.06.2019 09:10

Mathematics, 28.06.2019 09:10








Mathematics, 28.06.2019 09:10

Biology, 28.06.2019 09:10



Mathematics, 28.06.2019 09:10

Biology, 28.06.2019 09:10

Mathematics, 28.06.2019 09:10

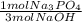 = 1.25 mol Na₃PO₄
= 1.25 mol Na₃PO₄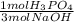 = 1.25 mol H₃PO₄
= 1.25 mol H₃PO₄

