57.34
D
zoom in
4
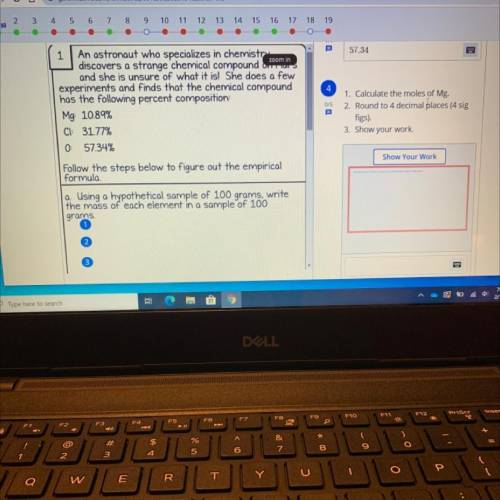
1 An astronaut who specializes in chemistry
discovers a s...

Chemistry, 13.02.2021 05:10 sanfratello1081
57.34
D
zoom in
4
1 An astronaut who specializes in chemistry
discovers a strange chemical compound Om
and she is unsure of what it is! She does a few
experiments and finds that the chemical compound
has the following percent composition
Mg: 10.89%
CI: 31.77%
0 57.34%
0/5
1. Calculate the moles of Mg.
2. Round to 4 decimal places (4 sig
figs).
3. Show your work.
Show Your Work
Follow the steps below to figure out the empirical
formula
a Using a hypothetical sample of 100 grams, write
the mass of each element in a sample of 100
grams
7:05 PM
2/12/2021
D
9
RI
Type here to search

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2


Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Questions

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Chemistry, 15.12.2020 01:00




History, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Spanish, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Health, 15.12.2020 01:00



English, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00




